Background: The Belgian ibrutinib Real-world Data (BiRD) study is a multicenter, non-interventional, observational cohort study of adults with confirmed CLL, mantle cell lymphoma, or Waldenström's macroglobulinemia.
Aim: To assess the effectiveness and safety of ibrutinib treatment of CLL in routine clinical practice in Belgium.
Methods: Eligible patients initiated reimbursed ibrutinib treatment upon or after commercial availability in Belgium (August 1, 2015) or participated in the Medical Need Program and switched to reimbursed ibrutinib with commercial availability. The primary outcome measures were progression-free survival (PFS) and overall response rate (ORR); secondary outcome measures included overall survival (OS) and safety. Data were collected both prospectively (pro) and retrospectively (ret). Pro-collected adverse events (AEs) included all treatment-emergent AEs (TEAEs), whereas ret-collected AEs included only those considered related to ibrutinib; therefore, TEAEs for the pro group and AEs for the ret group are reported separately. To account for patients who could not be included in the ret group as they died before study entry and were therefore unable to provide consent, total population effectiveness results were adjusted with left truncation. We report results from the fourth interim analysis (cutoff: August 20, 2022) for patients with CLL, with a median follow-up of 49.1 months.
Results: The effectiveness population included 221 patients (pro, n = 71; ret, n = 150). Median age at ibrutinib initiation was 72 years (range, 38-90); 64.3% were male; median time from diagnosis to ibrutinib initiation was 5.8 years (range, 0-31.4). Del17p/ TP53 mutation was present in 78/132 (59.1%) patients; 22/80 (27.5%) were IGHV-mutated. Nearly one-third of patients (27.4%) were treated first line, 24.2% received 1 prior line of therapy (LOT), 27.9% received 2 prior LOTs, and 20.5% received ≥ 3 LOTs. A history of significant cardiovascular disease was reported by 17.8% patients. Ibrutinib was initiated at 420 mg/day in 88.1% of patients, and 91 (40.3%) patients had ≥ 1 dose modification. Among these patients, who may have had more than 1 modification (increase or decrease), the most common reasons for dose modification were physician preference (59.3%) and toxicity (45.1%). Of the total 226 patients, 58 (25.7%) had ≥ 1 dose increase and 89 (39.4%) had ≥ 1 dose decrease.
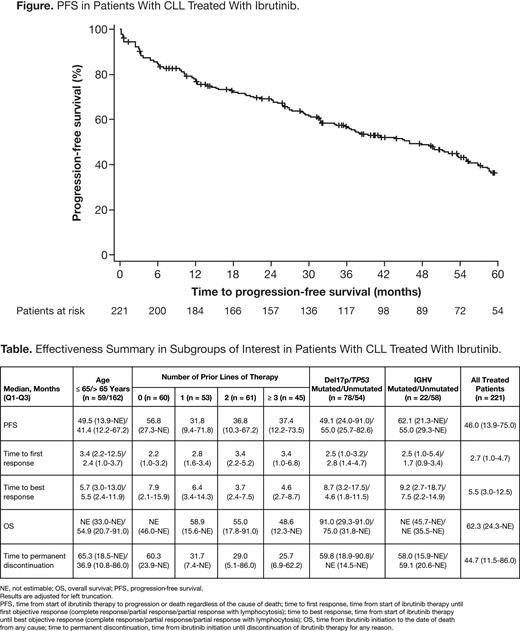
With ibrutinib treatment, median PFS was 46.0 months in the overall CLL population (Figure), and 49.1/53.6 months in the pro/ret groups. ORR was 90.9% (CR 21.7%, PR 53.4%, PR with lymphocytosis 15.8%). Median OS was 62.3 months (not estimable/91.0 months in pro/ret). Main effectiveness outcomes stratified according to age, prior LOT, and mutation status are listed in the Table.
The safety population included 226 patients (pro, n = 73; ret, n = 153). TEAEs/AEs and serious TEAEs/AEs were reported in 100.0/85.6% and 69.9/50.3% in the pro/ret groups, respectively. TEAEs/AEs led to dose reduction, interruption, or withdrawal in 24.7/13.7%, 45.2/28.8%, or 30.1/21.6% of patients in the pro/ret groups, respectively. TEAEs/AEs of interest in the pro/ret groups were major bleeding (5.5/2.6%), infection (76.7/65.4%), hypertension (17.8/11.8%), atrial fibrillation (13.7/9.2%), arthralgia/myalgia (27.4/11.1%), diarrhea (24.7/13.7%), and rash (16.4/9.8%). Ibrutinib was permanently discontinued in 164 (72.6%) patients, most commonly due to disease progression (n = 40). The median duration of treatment was 35.8 months; 32 of the 226 patients discontinued ibrutinib due to toxicity (14.2%), and 27.4% of patients remain on treatment.
Conclusions: In this Belgian real-world study, ibrutinib was found to be an effective treatment for patients with CLL, including those with higher risk mutations, several prior LOTs, and older age, with first-line patients who joined the study later responding particularly well. These findings are consistent with those from clinical trials, which support more effective outcomes for ibrutinib when utilized in the frontline (eg, RESONATE-2: Barr PM, et al. Blood Adv. 2022;6:440-3450; RESONATE: Munir T, et al. Am J Hematol. 2019;94:1353-1363). TEAEs/AEs are consistent with the known safety profile of ibrutinib.
Disclosures
Saevels:Galapagos: Research Funding. Maes:Janssen: Current Employment. Lahaye:Janssen: Current Employment. Janssens:AstraZeneca: Other: Advisory board, Speakers Bureau; Beigene: Consultancy, Other: Advisory Board, Speakers Bureau; Sanofi: Consultancy; Novartis: Other: Advisory Board, Speakers Bureau; Janssen: Consultancy, Other: Advisory Board, Travel, Speakers Bureau; Amgen: Consultancy, Other: Advisory Board, Travel Grants, Speakers Bureau; Abbvie: Consultancy, Other: Advisory Board, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal